 HKU Department of Earth Science
HKU Department of Earth Science
Seminar
Interactions between aqueous transition metals (Au, Zn) and organic molecules probed by ESI mass spectrometry and density functional theory
-
Date
July 10,2015
-
Time
11:00AM
-
Venue
JL314A
-
Speaker
Ms. Magdalena Anna Taczhowska Department of Earth Sciences, HKU
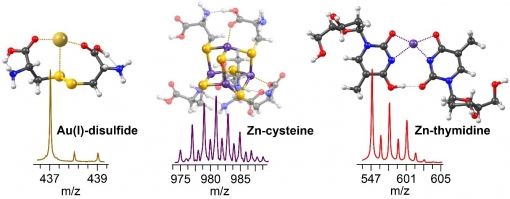
The primary objective of this study was the application of ESI FT-ICR mass spectrometry and DFT calculations in the investigation of molecular metal-biomolecule complexes. Three independent ESI-MS experiments were performed, in which aqueous solutions of AuCl3:cysteine, ZnCl2:cysteine and ZnCl2:thymidine were analyzed. In the case of gold-cysteine system, the formation of disulfides and gold(I)-disulfide species was observed. Structural and energetic data of Au(I) complexes were calculated at the M06 level theory, and using TD-DFT and PCM/TD-DFT computations, UV-Vis properties of these species were predicted. The major goal of the second project was to deliver speciation, structures and stability of aqueous zinc-cysteine complexes. ESI-MS of ZnCl2:cysteine solution resulted in formation of mono- and polynuclear Zn complexes. DFT calculations further showed that tetranuclear Zn-cysteine species can be considered as potential intermediates during sphalerite formation. In the third study the focus was on the complexation behavior of zinc with nucleoside thymidine. IRMPD-MS analysis of the most abundant [Zn(dT)(dTH)]+ showed that the most favorable dissociation channel for zinc-thymidine complexes is the neutral removal of 2’-deoxyribose and the subsequent fragmentations proceed via cross-ring bond raptures of thymine. The M06 calculations showed that zinc-thymidine complexes retain symmetric structures and their predicted gas-phase stability was found to reflect ESI-MS experimental results.
In each project the ion abundance and distribution of gaseous metal-free and metal-containing species were found to be critically dependent on the i) solution concentration; and ii) time, at which feed solution was electrosprayed. Therefore, from a geochemical perspective, ESI mass spectrometry is considered as an excellent tool for delivering new insights into metal speciation in bulk solution.
